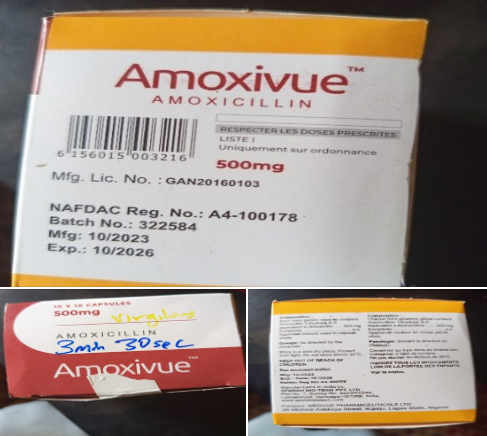
NAFDAC is alerting the public to the recall of Amoxivue (Amoxicillin) 500mg, with NAFDAC No. A4-100178 and Batch No. 322584, manufactured by Sparsh Bio-Tech Pvt, Ltd.
In a statement released today August 7, the agency said a batch of Amoxivue (Amoxicillin) 500mg capsules, manufactured in 10/2023 and expiring in 10/2026, was sampled from a facility in Sokoto and two facilities in 2 Local Government Areas in Plateau State.
The agecy said the capsules were analysed using High-Performance Liquid Chromatography (HPLC) and Fourier Transform Infrared Spectroscopy (FTIR). The laboratory analysis report showed that the API content was significantly low at 26.3%.
‘’The weight variation and infrared absorption spectrum of the sample residue did not meet the established specifications. This indicates that the product is substandard, which has led to its recall.
Amoxivue (Amoxicillin) 500 mg capsules is an antibiotic used to treat various bacterial infections, including respiratory tract infections, ear infections, sinus infections, urinary tract infections, and skin and soft tissue infections. It contains amoxicillin, a penicillin-type antibiotic that works by inhibiting the growth of bacteria.
Risk Statement
Administration of Amoxivue (Amoxicillin) 500 mg with low levels of amoxicillin may lead to therapeutic failure, antibiotic resistance, increased risk of complications, misleading clinical assessments, and poses a risk to public health.”the statement reads in part
The agency said healthcare professionals and consumers are advised to report any suspicion of the sale of substandard and falsified medicines or medical devices to the nearest NAFDAC office, call 0800-162-3322 or send an email to [email protected]



Now Playing: Love Bug
Aretti Adi
SPONSORED LINKS
LOAN FOR TRAVEL, VISA, JAPA, PoF UP TO N200M (CLICK HERE)
[CLICK HERE] For Music Artwork, Website Design And SEO Setup
INSTALL 9JAFLAVER MUSIC APP, STREAM, DOWNLOAD, AND PLAY MUSIC OFFLINE
CHECK OUT FUNNY PICTURE AND MEME HERE (CLICK HERE)
Chissom Anthony – Glory To God In The Highest [See Trending Gospel Song]
Copyright © 2014-2026 9jaflaver. All Rights Reserved.
About us | DMCA | Privacy Policy | Contact us
| Advertise| Request For Music | Terms Of Service
9jaflaver is not responsible for the content of external sites.